Research focus: Hematopoietic stem cells
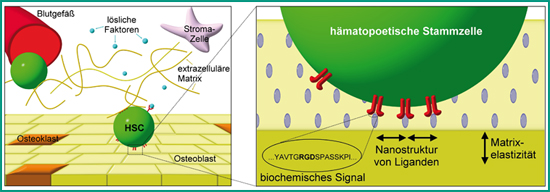


Hematopoietic stem cells (HSCs) give rise to all types of blood cells. Up to now, they cannot be expanded in vitro without losing their stem cell character. In the body they are maintained in specialized microenvironments, so-called stem cell niches. The aim of our group is to develop and test biomaterials, which mimic the HSC niche in regard to its specific ligands, micro- and nanostructure, dimensionality as well as mechanical properties. We study the interaction of HSCs with these biomimetic materials in order understand how the physical and mechanical properties of the environment influence HSCs. The final goal of these studies is to create an artificial stem cell niche for the in vitro proliferation of HSCs.
Research project: BioNanoHSCs
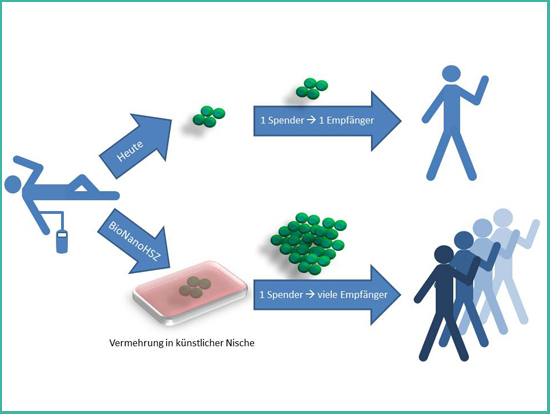


Figure 2. Principle of the project BioNanoHSC.
The project BioNanoHSC is funded by the NanoMatFutur program of the German Federal Ministery of Education and Research (BMBF) with 3.18 million € (FKZ: 13N12968). The acronym BioNanoHSC stands for “Biomimetic Nanostructured Materials for Culturing Hematopoietic Stem Cells - BioNanoHSC”.
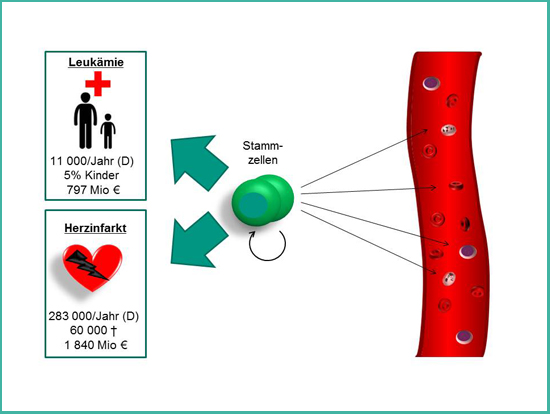
Hematopoietic stem cells are the stem cells of the blood system and supply us during our entire life with fresh blood cells. Diseases of the blood system such as leukemia can be treated since the 1960s by replacing the diseased blood system with a healthy one by transplantation of stem cells or bone marrow from a healthy donor. Furthermore, hematopoietic stem cells hold great promise for the future treatment of patients after myocardial infarction – one of the leading causes of death in Germany.
An elegant approach to cover the demand of all patients who would profit from a treatment with hematopoietic stem cells is the multiplication of these stem cells in the laboratory. Today, this is not possible with a satisfying outcome. The only place where hematopoietic stem cells can multiply without restriction is their natural environment – their niche in the bone marrow. This niche is uniquely specialized for maintaining stem cells. New scientific discoveries show that material properties of the environment have a profound impact on cells. Therefore, the aim of the project BioNanoHSC is to study how stem cells interact with innovative nanostructured materials. The gained knowledge will be used to develop new materials with tunable material properties that mimic the natural environment of stem cells. These artificial niches will help to achieve the goal of a targeted multiplication of hematopoietic stem cells, which would be a great benefit for tens of thousands of patients.
ERC Starting Grant: bloodANDbone
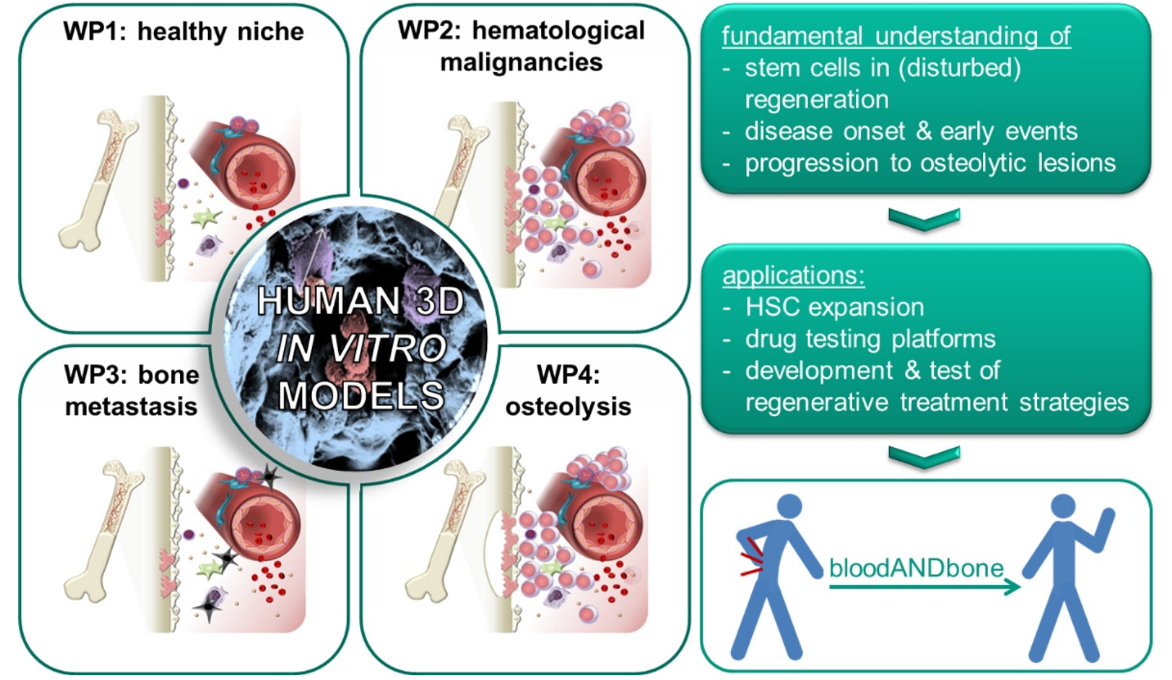
Blood and bone are closely intertwined. Their intrinsic regenerative capacities are disturbed in many hematological and musculoskeletal diseases. Re-establishing the regenerative potential is the key to cure these diseases by regenerative medicine. Multipotent stem cells of both tissues – hematopoietic stem cells (HSCs) for blood and mesenchymal stem/stromal (MSCs) for bone – are the basis for their regenerative capacity. While it is well established that HSCs are influenced by the bone marrow in their natural environment including MSCs and their progeny, surprisingly little attention has been paid to the reciprocal relationship. The hypothesis of the ERC Starting Grant project bloodANDbone is that only when taking both tissues and their mutual crosstalk into account, we will be able to understand how the regenerative potential of blood and bone is impaired in disease and how it can be re-established with novel treatment strategies. For this purpose we need to understand the early events of disease onset and progression. Due to the limitations of such studies in human beings and animals, we will develop human in vitro models of healthy bone marrow, which can be induced to develop hematological and musculoskeletal diseases with high incidence, namely leukemia, multiple myeloma and bone metastasis. Previously my team and I developed a simplified bone marrow analog that bases on macroporous, cell-laden biomaterials with tunable physical, biochemical and biological properties. This versatility will enable us to create biomimetic human in vitro models of the human bone marrow in health and disease, which will allow us to investigate how the regenerative balance of bone marrow is maintained in health and disturbed in the different kinds of diseases – a prerequisite to develop novel regenerative treatments – and to establish in vitro test systems for screening of novel drugs or treatments.
Biomaterials for the culture and targeted differentiation of iPS cells
The induced pluripotent stem cell (iPSC) technology was discovered by Shinya Yamanaka’s lab in Kyoto, Japan. They inserted four specific genes encoding transcription factors which could convert adult cells into pluripotent stem cells. iPSCs are capable to differentiate into all adult cell types and self-renewal indefinitely. Therefore they are a potential cell source for regenerative medicine applications and in-vitro disease models. To date, culture substrates coated with Matrigel™ have been presenting promising results for iPSC culture and differentiation. Although Matrigel™ provides an improvement over standard monolayer cultures, it provides some drawbacks such as batch to batch variation and decrease of cytochromic activity of the cells. As an alternative, engineered biomaterials can be fabricated by adjusting biological and biophysical characteristics to maintain iPSCs culture.
The aim of this BMWi funded project (ZF4397302CS7) is to develop biomaterials that help to fabricate a) a fully synthetic Matrigel™-mimetic hydrogel for “feeder-cell free culture” that allows investigating the influence of biophysical and biochemical parameters on iPSCs and b) biomimetic gels that mimic on the one hand the hepatic progenitor cell (HPC) niche or the hepatocyte environment for the targeted differentiation an culture of hepatic cells.
Contact

 © Markus Breig, Karlsruher Institut für Technologie (KIT)
© Markus Breig, Karlsruher Institut für Technologie (KIT)
30419 Hannover

 © Markus Breig, Karlsruher Institut für Technologie (KIT)
© Markus Breig, Karlsruher Institut für Technologie (KIT)